Calorimetry refers to the measure of how much heat is transferred in a reaction or phase change. We measure this heat with a device called a calorimeter. Although serious chemists will use serious calorimeters costing hundreds or thousands of dollars, you can duplicate much of their work by pouring some water into a Styrofoam cup. Your answers won’t be as precise as the serious chemists’ answers, but for two or three sig figs, this method is good enough.
There are two kinds of calorimetry problems; we’ll look at one here and the other in the next post. The first involves dropping a heated block of metal into the cup of water. The block cools down and the water warms up until the block and water are at the same temperature (thermal equilibrium). In the second type of problem, a chemical reaction takes place in the water. If the reaction is exothermic, the water absorbs the heat and warms up. If the reaction is endothermic, heat is absorbed from the water and the water cools down.
Let’s examine the first problem: There are two guiding principles we observe. The first is that heat will flow from the hot block into the water until the block and the water are at the same temperature. The second principle is that (we assume) there is no loss of heat to the air. In other words, the heat lost by the block exactly equals the heat absorbed by the water.
Both the heat lost by the block and the heat the water absorbs are governed by a simple equation:

Here q is the amount of heat gained or lost, m is the mass of the substance, c is the specific heat capacity of the substance, and ΔT is the change in temperature. Many teachers call this the M-CAT equation because the Δ looks like an A if you squint really hard. If this helps you remember it, you can call it that too. The value of c, the specific heat capacity, is a constant for a given substance. It measures how much heat must be absorbed by 1 g of a material to raise its temperature by 1°C. You might be asked to memorize the specific heat capacity of water. It is 1.00 cal/g·°C or 4.18 J/g·°C. You probably will not need to memorize specific heat capacity values for any other substance.
With the equation, calorimetry problems of this type are simple algebra problems. We have  and
and 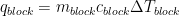 . Also, since the heat lost by the block equals the heat gained by the water, we can write
. Also, since the heat lost by the block equals the heat gained by the water, we can write  . Therefore,
. Therefore,
 .
.
This is our magic equation. There are two kinds of questions you will answer with this equation. In one, we tell you the initial and equilibrium temperatures and you calculate the specific heat capacity of the block substance. In the other, you are given the initial temperatures and the specific heat of the block and you calculate the equilibrium temperature. Here are examples that show both types:
Example 1: A 25 g block of aluminum is heated to 85°C and placed into a calorimeter with 80 g of water at 25°C. The final temperature of the calorimeter and block is 28.8°C. Calculate the specific heat of aluminum.
Solution 1: We calculate the ΔT of the water (3.8°C) and the aluminum block (-56.2°C) and plug everything into the equation above.
(80)(4.18)(3.8) = -(25)(c)(-56.2). Solving gives c = 0.90 J/g·°C.
Example 2: A 25 g block of aluminum is heated to 85°C and placed into a calorimeter with 80 g of water at 25°C. The specific heat of aluminum is 0.90 J/g·°C. What is the equilibrium temperature of the calorimeter?
Solution 2: This problem (really just a rewording of example 1) is only a little more complicated to solve than example 1. First note that ΔT = Tf – Ti. We make this substitution and our magic equation becomes
(80)(4.18)(Tf – 25) = -(25)(0.90)(Tf – 85)
Distribute and simplify to get 334.4 Tf – 8360 = 1912.5 – 22.5 Tf. Solving gives Tf = 28.8°C.
Note: This is one of the rare times you do not need to convert the temperature from °C to kelvins. This is because the temperature term in the equation is a change in temperature and not an absolute temperature. Changing the temperature by 1°C is the same as changing it by 1 K.
